
Modern lasers come in an incredible range of different designs, operating at different wavelengths, different power levels, and using different materials as the basis for stimulated emission. It’s amazing to imaging after 60 years, but even after lasers were created, many were unsure what to do with the device; as Charles Hard Townes later reflected, “Many people said to me—partly as a joke, but also as a challenge—that the laser was ‘a solution looking for a problem.’”
Though its usefulness may have initially be somewhat unclear, the laser is now a tool in countless applications, industries, and fields of study. In 1964, Townes won the Nobel Prize in Physics with Nikolay Basov and Aleksandr Prokhorov for “fundamental work in the field of quantum electronics, which has led to the construction of oscillators and amplifiers based on the maser-laser principle.”
But what exactly is a laser, and how does it work? Now we can be a little more specific about the physics. Most people are familiar with the orbital model of the atom introduced by Niels Bohr in 1913, and illustrated below.
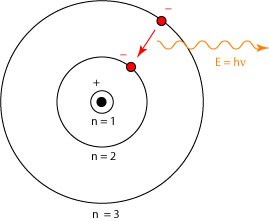
Researchers had long observed that atoms and molecules appear to only absorb and emit light at certain frequencies, giving them a characteristic spectrum of emission. Bohr argued that this must arise because the electrons in an atom can only orbit the nucleus at certain special distances, labeled in the picture by an index “n” for convenience. The innermost orbit is the lowest energy state, or “ground state.” An atom can only emit a photon when one of its electrons drops from a higher energy state to a lower energy state, and can only absorb a photon but jumping from a lower energy state to a higher energy state: a “quantum leap.”
For all but the simplest atoms and molecules, there are many, many possible energy levels, and a photon with the appropriate energy can cause them to jump between any pair of levels. If we picture energy levels as physical altitude, we can draw a sketch of Einstein’s three processes of photon-atom interaction as shown below.
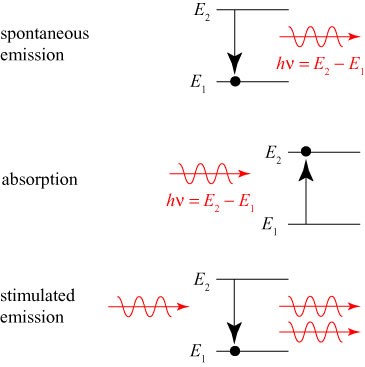
In spontaneous emission, the atom drops from level 2 to level 1, releasing a photon with energy equal to the difference in energy levels. In absorption, the reverse happens. In stimulated emission, a photon interacts with the atom, causing it to release an identical photon.
As we have noted, this process of stimulated emission is what makes the laser work. If we put enough atoms into the excited state, so that they can experience stimulated emission, then the two photons released in one stimulated event can cause two additional stimulated emissions, and so forth. The result is an avalanche of photons, all identical.
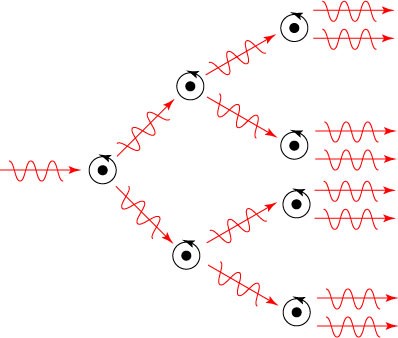
But the challenge is, in fact, getting a large number of atoms into the excited state in the first place. If we are only working with our two energy levels, level 1 and level 2, then we can at most get 50% of our atoms into the excited state. At that point, any photon passing through the system is equally likely to be absorbed or causing a stimulated emission. But this prohibits our avalanche from starting: all the unexcited atoms effectively put the brakes on the process.
The solution is to design our laser to take advantage of more than two energy levels of the atom, as shown below.
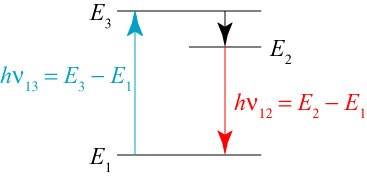
Let us suppose that such a 3-level atom that is excited to level 3 very quickly decays to level 2, but the atom takes a relatively long time to decay back to the ground state, level 1. If we excite a collection of these atoms to level 3 using a light beam at frequency , they will drop to level 2 too fast to be de-excited by the light beam! Any atoms still in level 1 can then be also excited to level 3, and so on. By this process, we end up with most of the atoms continually in level 2, and almost no atoms in level 1: we have achieved what is called population inversion, a key ingredient to making a laser work.
A laser is more than stimulated emission — a number of components are required to make a functioning device. In fact, the “avalanche” of photons we described above is a distinct phenomenon with its own name, amplified spontaneous emission: an initial photon created by spontaneous emission produces many more photons by inducing stimulated emission in excited atoms.
So what makes a laser? In simplified form, a true laser consists of four components, listed and illustrated below.
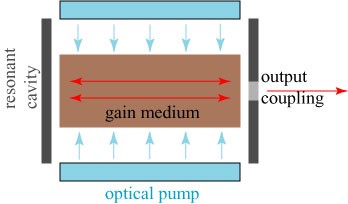
It is appropriate to refer to the lasing process as an avalanche, because the invention of the laser itself has had an avalanche effect on society. From the first crude prototype of Townes, the laser has become a ubiquitous part of even our daily lives. In telecommunications, infrared lasers are used to send information through fiber optic cables. Laser printers use near infrared light to trace patterns on paper. Scanners at the supermarket use red lasers to read product barcodes, and we use red lasers to entertain our cats. Green lasers are popularly used as laser pointers in office presentations. The optical disk that stores games on a Playstation or Xbox gaming console reads the data using a blue laser. Ultraviolet lasers, whose light is readily absorbed by organic compounds, are used in eye surgeries such as LASIK. The uses of lasers in scientific research are truly too numerous to mention.

Latest Comments
Have your say!