
‘Oh, I’ve cut out carbs. I feel so much healthier’, many people tell me. But I doubt they know what ‘carbs’ really are, nor why we really need them in our diet. The word carbohydrate came in during the 19th century. It was used to refer to materials whose molecules had the form Cn(H2O)n. C indicates a carbon atom, H and O hydrogen and oxygen respectively; n is almost any number. To illustrate, glucose has the molecular formula C6H12O6 – or it could be represented C6(H2O)6., leading early chemists to think that carbohydrates were compounds made of carbon (C) and water (H2O). That’s not quite so, but it will help us understand their chemical properties.
Like many substances in metabolism, carbohydrates can take the form of polymers – substances whose molecules are composed of many smaller molecules joined together. These smaller units are the sugars, which often have names indicating their original source: glucose, grape sugar; fructose, fruit sugar; lactose, milk sugar for instance. (The convention of naming sugars with an ‘ose’ also comes from the 19th century. It is said that when the Hungarian biochemist Albert Szent-Györgyi first isolated Vitamin C – for which he was awarded the Nobel Prize in Physiology or Medicine in 1937 – thinking that it had sugar-like properties, but not knowing its structure, he suggested it be called ‘Godnose’.) The molecules of ‘simple sugars’ such as glucose and fructose (each of which has the formula C6H12O6) can join together, making substances called disaccharides (‘two sugars’): the disaccharide composed of glucose and fructose is called sucrose, and is what we recognise as table sugar. Lactose, the sugar found in milk, is a disaccharide composed of glucose and the sugar galactose (which, as it happens, also has the formula C6H12O6).
But this joining up doesn’t stop there. Glucose especially can be joined in long chains of glucose molecules. They may be straight chains – each glucose molecule joined to one either side – or they may have branches, where a branch heads off from a third position on one of the glucose molecules. These big molecules, with potentially several thousand individual glucose molecules, are what starch is. Starch is a storage material made by many plants. It forms the basis of everything we think of as starchy foods, or indeed carbohydrates.
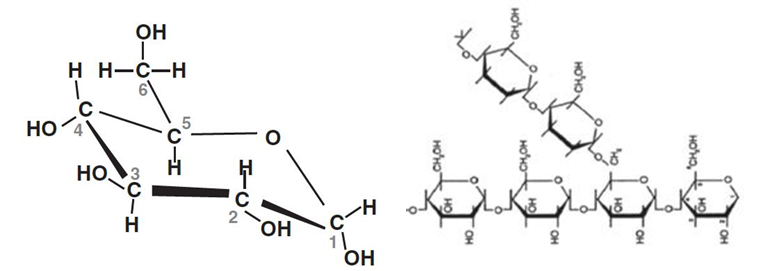
When we eat starchy foods, the bonds between the individual glucose molecules are broken down as the food is digested, and eventually, in our small intestine, glucose can be taken up and transported into the bloodstream. Glucose is by far the major sugar in our bodies and is a ready source of energy for tissues such as the brain and our muscles. But – although I might want to carry on thinking after a meal – I might not want to go out and exercise immediately. So we need to store spare glucose. And we do that by making our own form of starch, called glycogen. (Glycogen and starch are very similar in structure, but with one difference: each large molecule of glycogen is built around a protein molecule called glycogenin.) We store glycogen in our liver cells and in our muscles. Typically we store around 100 g of glycogen in the liver when we have eaten. If we then eat nothing else, it all disappears within 24 h. And it so happens that the brain needs about 100 (maybe 120) g of glucose in every 24 hours. Our glycogen store in the liver is a buffer to keep the brain going if we don’t, or can’t, eat. (Beyond that, other things happen to keep us alive – to be discussed in a later blog.) When we need to use that glycogen, enzymes break off individual glucose molecules which can be used in the same cell, or transported to other tissues via the bloodstream.
Now, I hinted earlier about particular chemical properties of carbohydrates. Because of their molecular makeup, they tend to mix easily with water: indeed, glucose and other simple sugars all dissolve readily in water (or tea or coffee). That gives them a special role in metabolism: they are readily transferred between tissues, via the bloodstream. The situation is very different from fats, which don’t dissolve in water, and require special systems to be transported around the body. When you start to exercise, your muscles initially use glucose (it may come directly from the glycogen in the muscles). Your brain will, in normal circumstances, use almost entirely glucose for its energy supply. But – because carbohydrates mix with water – they are heavy to store. Each gram of glycogen is stored with around 3 g of water. Given that our livers typically weigh around 1.5 kg, you can see that a full glycogen store (say 100 g glycogen plus 300 g water) makes it quite a bit bigger – stretches the cells. That’s why fats are needed as our longer-term energy store. More on that next time!

Author: Keith Frayn
Title: Understanding Human Metabolism
Paperback ISBN: 9781009108522
Latest Comments
Have your say!